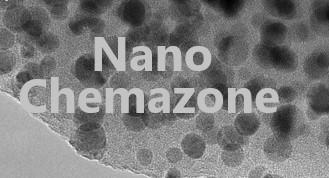
Monolayer Tungsten Disulfide (WS2) Quantum Dots
₹0.00
Monolayer Tungsten Disulfide (WS2) Quantum Dots
| MF: | WS2 |
| Chemical Name: | Tungsten Disulfide |
| Purity: | > 99.99% |
| Size: | ≤ 10 nm (Size Customization possible) |
| Concentration: | 0.5 to 1 mg/ml (Customization possible) |
| Form: | Powder/Dispersion |
| Product Number: | NCZAM110 |
| CAS Number: | 12138-09-9 |
Monolayer Tungsten Disulfide (WS2) Quantum Dots
Note: For pricing & ordering information, please contact us at sales@nanochemazone.com
Please contact us for quotes on Larger Quantities & Customization. E-mail: contact@nanochemazone.com
Customization:
If you are planning to order large quantities for your industrial and academic needs, please note that customization of parameters (such as size, length, purity, functionalities etc.) are available upon request.
Related products
Colloidal Silver Solution Antimicrobial Properties
Silver Nanoparticles Dispersion
Colloidal Silver Dispersion
Silver Dispersion Nanoparticles
| MF: | Ag |
| Chemical Name: | Colloidal Silver Nano Water Dispersion |
| Purity: | > 99.99% |
| APS: | <40 nm (Size Customization possible) |
| Form: | Colloidal Nano dispersion |
| Product Number: | NCZD1004-20 |
| CAS Number | 7440-22-4 |
| Concentration | 0.02 mg/mL (Customizable) |
The antibacterial effects of silver nanoparticles have been used to control bacterial growth in a variety of applications, including dental work, surgery applications, wounds and burns treatment, and biomedical devices. It is well known that silver ions and silver-based compounds are highly toxic to microorganisms. Silver nanoparticles (AgNPs) are considered as a promising antibacterial agent, antifungal, antiviral properties and always used to modify orthopedic implants to prevent infection.
Molybdenum Disulfide (MoS2) Quantum Dots
Monolayer Molybdenum Disulfide, MoS2
Monolayer Tungsten Disulfide Powder, WS2
Nano Size Monolayer Tungsten Disulfide, WS2
Nanosize Monolayer Molybdenum Disulfide, MoS2
Silver Nanoparticles/Nanomaterials
Silver Nanopowder
Silver Nanoparticles
Nano Silver Powder
| MF: | Ag |
| Chemical Name: | Silver Nanoparticles |
| Purity: | > 99.99% |
| APS: | 50 nm (Size Customization possible) |
| Form: | Nanopowder/Dispersion |
| Product Number: | #NCZ5201 |
| CAS Number | 7440-22-4 |
Applications:
The antibacterial effects of silver nanoparticles have been used to control bacterial growth in a variety of applications, including dental work, surgery applications, wounds and burns treatment, and biomedical devices. It is well known that silver ions and silver-based compounds are highly toxic to microorganisms.
Silver Nanoprism
Silver Nanoprism
Silver Nanoplate
| MF: | Ag |
| Chemical Name: | Silver Nanoprism |
| Purity: | > 99.99% |
| APS: | <40 nm (Size Customization possible) |
| Form: | Aqueous Nano Prism dispersion in 5 mM sodium borate |
| Product Number: | NCZD1004-22 |
| CAS Number | 7440-22-4 |
| Concentration | 0.02 mg/mL (Customizable) PVP Functionalized |
| Wavelength | 400 nm to1060 nm (Customizable) |
Note: We supply different size products of micro and Nano Size range dispersion according to the client’s requirements.
Description: Halide modified aqueous solution of silver plates with hexagonal and triangular morphologies have large light absorption and scattering cross sections and broadband plasmonic peaks. These particles have average thicknesses of only 5nm and with plasmonic peaks that, depending on the size of the particles, span the visible to IR regions of spectrum. These particles are suitable for bio-molecular detection, surface enhance Raman spectroscopy and photo-thermal cancer therapies.
Appearance : Colorful (yellow to violet) Particle Shape : 2D Triangular Plates Plasmonic Peaks : 400 - 900 nm Particle Size : thickness: 5 nm, width: 30 - 100 nm Size distribution : Strongly dependent on the size Concentration : 0.1 mM of Ag Solvent : Water Absorption : 2 OD/cm CAS Number : 7440-22-4
References : Frank, A.; Kitaev, V. Chemical Communications 2009, 46, 7170‐7172 Frank, A. J.; Cathcart, N.; Maly, K. E.; Kitaev, V. J. Chem. Ed. 2010, 87, 1098‐1101